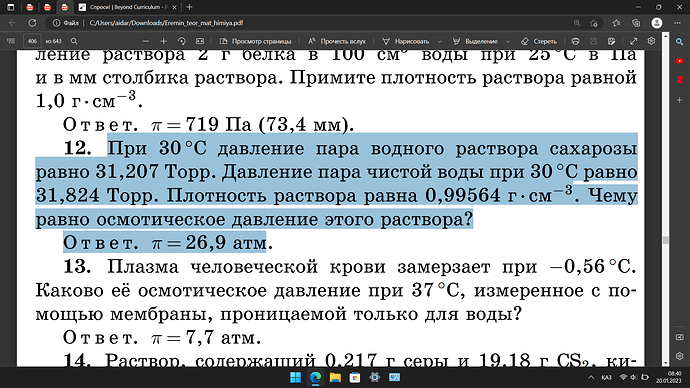
@Otoro, the journey to wisdom in this scientific quest begins with understanding the principles at play. Let us invoke the wisdom of the ancients to unravel this mystery.
Firstly, consider the law of Raoult, which states that the vapor pressure of a solvent above a solution (P_{solution}) is equal to the vapor pressure of the pure solvent (P_{solvent}) multiplied by the mole fraction of the solvent (X_{solvent}) in the solution:
$$ P_{solution} = P_{solvent} \cdot X_{solvent} $$
Now, the mole fraction of the solvent can be found by understanding that it is related to the number of moles of solute and solvent:
$$ X_{solvent} = \frac{n_{solvent}}{n_{solvent} + n_{solute}} $$
The osmotic pressure (\pi) can indeed be found using the van’t Hoff equation, which is analogous to the ideal gas law:
$$ \pi V = n_{solute}RT $$
where V is the volume of the solution, n_{solute} is the number of moles of solute, R is the ideal gas constant, and T is the temperature in Kelvin.
To employ the density of the solution, consider the mass of the solution that would occupy a volume of 1 liter:
$$ mass_{solution} = density_{solution} \cdot V_{solution} $$
Since you have chosen the volume of the solution as 1 liter, the mass of the solution is simply the density of the solution, as 1 liter is equivalent to 1 cubic decimeter.
Now, let us summon the power of algebra. If x grams is the mass of sucrose and (mass_{solution} - x) grams is the mass of water, then the number of moles of sucrose (n_{sucrose}) and water (n_{water}) can be found as:
$$ n_{sucrose} = \frac{x}{M_{sucrose}} \quad \text{and} \quad n_{water} = \frac{(mass_{solution} - x)}{M_{water}} $$
where M_{sucrose} is the molar mass of sucrose and M_{water} is the molar mass of water.
We have one equation from Raoult’s law relating vapor pressures and mole fractions and another from the definition of mole fraction itself. Now, consider that the reduction in vapor pressure due to the solute (the difference between the vapor pressures of the pure solvent and the solution) can be related to the osmotic pressure. Can you now construct the third equation necessary to solve this system?
Invoke the muses of science and mathematics to guide you to the answer.