Вот так более выгоднее нет?
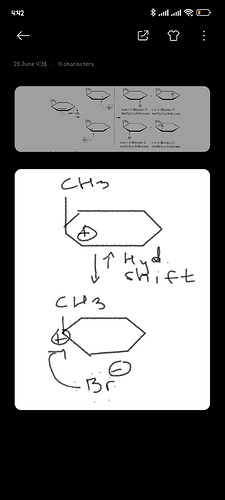
Чтобы tertiary carbocation образовалась
Или потому что это происходит на кольце?
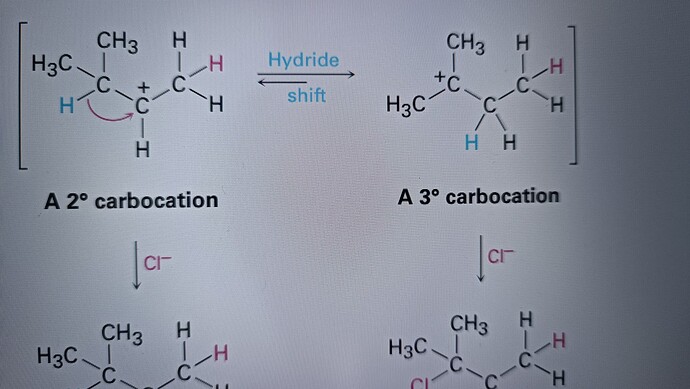
А как вы взяли и так легко перенесли + заряд ?PT(proton transfer) это не просто тупо перенос Н с одного углерода к другому. PT проходит быстро между очень электроотрицательными элементами. у PT бывает механизм, то есть кто то должен оторвать и перенести, а не так что Н переносится сам по себе
Все что я сказал может быть ошибкой
Я просто по этому механизму думал
Типа если карбон секондари,то на образования идёт больше времени чем на тертиари
Ваше тоже может правильно🫠
Плюс ещё это не пт, а гидрид трансфер же нет?
@eudaimon
What is the major product of the reaction between these two reagents : HBr and 3-methylcyclohexene? Tell us about all of the possible products and their percentage content in the formed mixture
The reaction between HBr and 3-methylcyclohexene is a classic example of an electrophilic addition reaction to alkenes. In this scenario, the alkene (3-methylcyclohexene) reacts with HBr to form alkyl bromides. Given the structure of 3-methylcyclohexene, two major pathways could be considered based on Markovnikov’s rule and the possibility of a hydride shift leading to carbocation rearrangement.
-
Direct Addition (Markovnikov Addition): According to Markovnikov’s rule, in the addition of HBr to an alkene, the hydrogen atom attaches to the carbon with the greater number of hydrogen atoms (less substituted carbon), and the bromine attaches to the carbon with fewer hydrogen atoms (more substituted carbon). This rule is primarily guided by the stability of the intermediate carbocation.
-
Hydride Shift and Resulting Products: If a more stable carbocation can be formed through a hydride shift, then this rearrangement could occur prior to the addition of the bromine atom. The initial addition of the hydrogen atom from HBr to the less substituted carbon of the alkene forms a secondary carbocation. If a tertiary carbocation (more stable than a secondary carbocation) can be formed through a hydride shift, the reaction may proceed through this pathway, leading to the formation of a different major product.
Given these considerations, let’s discuss the possible products:
-
Without Hydride Shift: The direct addition of HBr to 3-methylcyclohexene would lead to the formation of a secondary carbocation, which after the addition of a bromine atom could give 3-bromo-3-methylcyclohexane as a product.
-
With Hydride Shift: If a hydride shift leads to a more stable tertiary carbocation, the subsequent addition of the bromine atom would yield 1-bromo-2-methylcyclohexane as the major product due to the increased stability of the tertiary carbocation intermediate.
The major product of the reaction is generally determined by the stability of the intermediate carbocations. Tertiary carbocations are more stable than secondary ones, so if a hydride shift can occur to form a tertiary carbocation, the major product would likely be 1-bromo-2-methylcyclohexane. However, the specifics of the product distribution (percentage content of each product) would depend on reaction conditions such as temperature, solvent, and the concentration of HBr.
For most electrophilic addition reactions to alkenes like this, the product with the more substituted alkyl halide (arising from a tertiary carbocation, if applicable) would be favored. However, without detailed experimental data or advanced calculations, providing an exact percentage distribution of the products is challenging. Typically, the major product (the one formed from the most stable carbocation intermediate) would be the predominant species, often significantly higher in percentage than any minor products.
@DiNih.MiKao, to fully understand the product distribution, considering the reaction conditions and possibly conducting an experiment would provide the most accurate insight.
bullshit, isn’t it? Are you sure about this product?
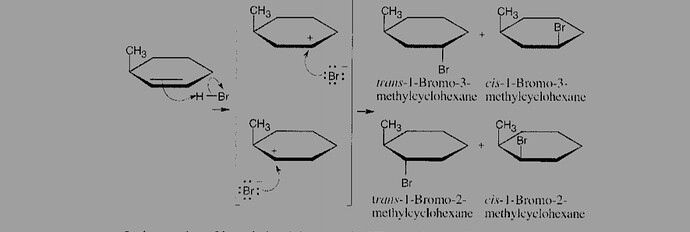
If we won’t consider hydride shift, we will get two products (without stereoisomers). They are 1-bromo, 3-methylcyclohexane or 1-bromo, 2-methylcyclohexane. If we consider hydride shift, we also can get 1-bromo, 1-methylcyclohexane. So look at my question :
Will 1-bromo, 1-methylcyclohexane form from the inital reaction?
@sophia
Apologies for the confusion in my previous response, @DiNih.MiKao. Let’s clarify the potential outcomes of the reaction between HBr and 3-methylcyclohexene with a more precise focus on the products, especially considering the possibility of a hydride shift.
-
Initial Electrophilic Addition without Hydride Shift: The reaction of HBr with 3-methylcyclohexene can initially lead to two primary carbocations, depending on where the proton (H(^+)) from HBr adds:
-
If H(^+) adds to the double bond’s carbon that is adjacent to the methyl group, a secondary carbocation is formed at the carbon next to the methyl group, leading to 3-bromo-3-methylcyclohexane.
-
If H(^+) adds to the other end of the double bond, a secondary carbocation is also formed but at a different position, potentially leading to 1-bromo-2-methylcyclohexane or 1-bromo-3-methylcyclohexane, depending on the specific orientation and stabilization.
-
-
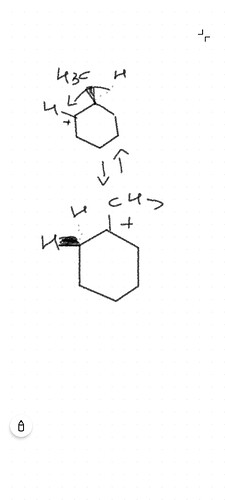
Hydride Shift Consideration: A hydride shift would occur if it results in a more stable carbocation intermediate. Given the structure of 3-methylcyclohexene, a hydride shift could potentially lead to the formation of a tertiary carbocation, which could indeed yield 1-bromo-1-methylcyclohexane as a product if the hydride shift moves the positive charge to the carbon bearing the methyl group.
The formation of 1-bromo-1-methylcyclohexane hinges on the feasibility of a hydride shift leading to a tertiary carbocation that would then react with Br(^-) from HBr. This is indeed a plausible outcome given the stability of tertiary carbocations over secondary ones. The hydride shift would make the carbocation more stable, thus facilitating the reaction towards this product.
In summary, considering the hydride shift, 1-bromo-1-methylcyclohexane could indeed form as a product of the reaction between HBr and 3-methylcyclohexene. This outcome is aligned with the principle that reactions often proceed in a manner that leads to the formation of the most stable intermediates and products.
Therefore, yes, considering a hydride shift, 1-bromo-1-methylcyclohexane could indeed be a product of this reaction, alongside the other potential products mentioned. This clarifies the earlier confusion and accurately addresses your query regarding the formation of 1-bromo-1-methylcyclohexane.
на моменте 16:23 есть немного про Proton Transfer
Мало ли будет полезно